Case Studies
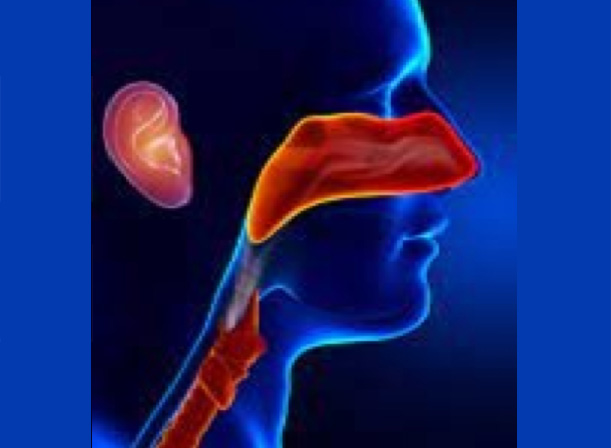
Case Study 1
Quadrant engaged with a large multinational medical device manufacturer to review their tracked tool designs in ENT applications.
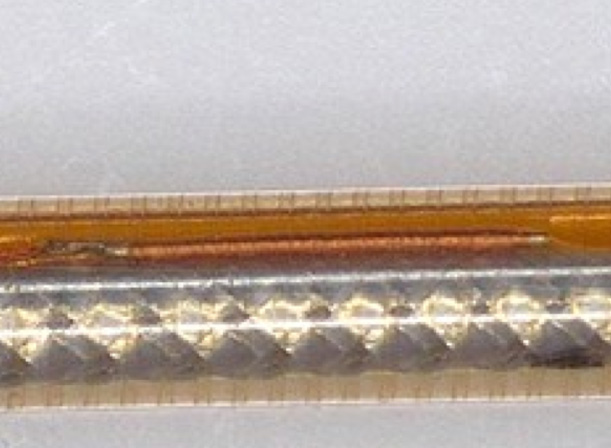
Case Study 2
Quadrant designed a customised sensor solution for endoluminal catheter deployment for an Irish SME.
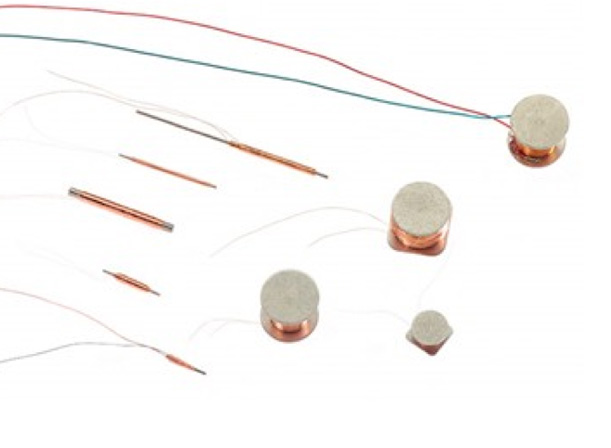
Case Study 3
Quadrant delivers characterisation and validation of novel sensor topology’s for a major supplier.
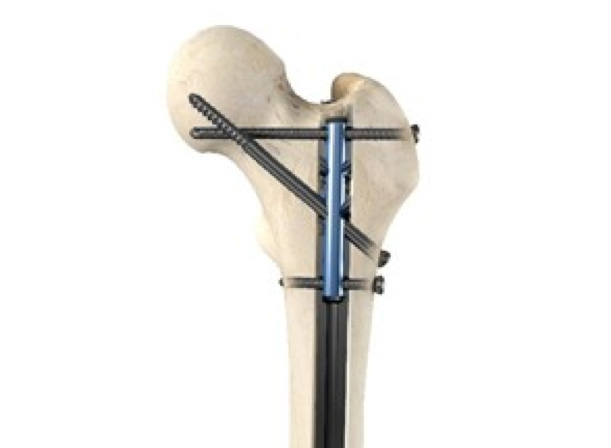
Case Study 4
Quadrant completed R&D validation for an Irish orthopaedics SME seeking to reduce fluoroscopy exposure during procedures.

Case Study 5
Quadrant completed extensive EMI compatibility testing for a NASDAQ listed client in the EP mapping segment.
Disclaimer
Quadrant Scientific navigation systems and tracking products are general metrology components that can be integrated into third party products, research experiments, or medical devices. The third party acquiring our system will be responsible to assign the specific intended use to their products and to identify the relevant risk classification of the final product incorporating Quadrant’s navigation system. The third party will be responsible for establishing the relevant regulatory, testing, verification and validation requirements and activities to prove safety and performance of the final product incorporating Quadrant’s technology. The third party remains responsible for any system-level testing and certification.
Contact
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.




